March 7, 2024
The six steps to designing a successful diversity action plan for a clinical trial protocol.

In December 2022, Congress passed the Diverse and Equitable Participation in Clinical Trials (DEPICT) Act1 which made it mandatory for sponsors to submit diversity action plans for clinical trials to the FDA early in the process. “Individuals from these populations are frequently underrepresented in biomedical research despite having a disproportionate disease burden for certain diseases relative to their proportional representation in the general population,” the agency commented.
The new legislation, along with growing industry awareness and motivation to right past wrongs, is changing the tide. While DEPICT allows for exemptions to the diversity plan requirement, trial sponsors must justify their waiver. And beginning this year, the FDA will publish, annually, an aggregate report of diversity action plans, along with the reasons any trials fell short of their set goals. This essentially creates accountability (albeit limited) for following through on diversity plans.
Some experts, however, say that the legislation doesn’t go far enough.
“Today’s policies and guidance are a start, but they should not be confused with being a mandate for diversity,” says Craig Lipset, founder of Clinical Innovation Partners and co-chair of the Decentralized Trials & Research Alliance (DTRA). “What is now required today is a diversity ‘plan,’ and the response for failure to deliver on that plan may be limited to requiring follow-up, real-world data studies. Such an outcome will only exacerbate there being a two-tiered system of evidence. There are opportunities to legislate more ‘carrots and sticks,’ as we know incentives drive action and commitment.”
Bridging the gap for small-to-mid-sized sponsors
While improving diversity in clinical trials is critical, it is not a simple endeavor—particularly for small-to-mid-sized biotechnology and startup digital therapeutics companies.
Global pharmaceutical companies have long faced the diversity issue, and in many cases, have dedicated significant time, funding, and personnel to begin to address it. Pfizer, for example, collaborated with the Tigerlily Foundation to create Health Equity Advocacy and Leadership (HEAL) workshops to better understand the breast cancer journey for women of color and foster inclusivity in clinical trials.2 The company also joined the #InclusionPledge—an initiative that aims to hold organizations accountable for taking specific actions to eliminate disparities for Black women living with breast cancer.
In addition, Johnson & Johnson is using artificial intelligence (AI) to increase diversity in 50 trials and plans to take that number to 100 in 2024, according to Najat Khan, PhD, J&J’s chief data science officer.3 The big pharma developed an internal platform called Trials360AI that leverages large de-identified datasets powered by AI and machine learning to help identify research sites with a high probability of enrolling diverse patients.
These behemoth organizations have a resource advantage and can leverage already-established diversity and inclusion programs, dedicated recruitment funding, and preexisting relationships with community outreach groups to support greater patient diversity within their clinical trials. But for other companies, the cost and logistics associated with recruiting and retaining a diverse cohort of participants can be daunting. Developing a diversity action plan adds a thick layer of up-front work that slows progress and increases costs—for instance, the expense associated with finding and engaging boutique partners who are experts in specialties such as minority recruitment.
One of the biggest challenges—for large and small companies—is retrofitting a protocol design already in progress to meet the new regulatory requirements. Not only is it time-consuming to reverse-engineer a diversity plan into an existing protocol but it’s also not always effective. Early planning is the key—even more so if the sponsor is planning on incorporating a virtual component to the trial. It is very difficult to “digitize” an existing protocol rather than building a protocol fit-for-purpose from the ground up with digital components baked in from the start.
The new FDA guidance says a diversity plan should outline the following:
- Enrollment goals, disaggregated by age group, sex, and racial and ethnic characteristics.
- Rationale behind these goals, including information about the condition and its prevalence among various groups.
- Outreach and enrollment strategies to accomplish these goals.
Some of the key strategies sponsors can employ include limiting exclusion criteria (the FDA has acknowledged its past guidance may have led sponsors to go overboard on their inclusion/exclusion criteria and is now trying to be more flexible); embracing digital tools to identify more participants based on demographics; and reducing the burden on participants by leveraging decentralized clinical trial (DCT) methodologies and technologies, such as eConsent and virtual trial sites.
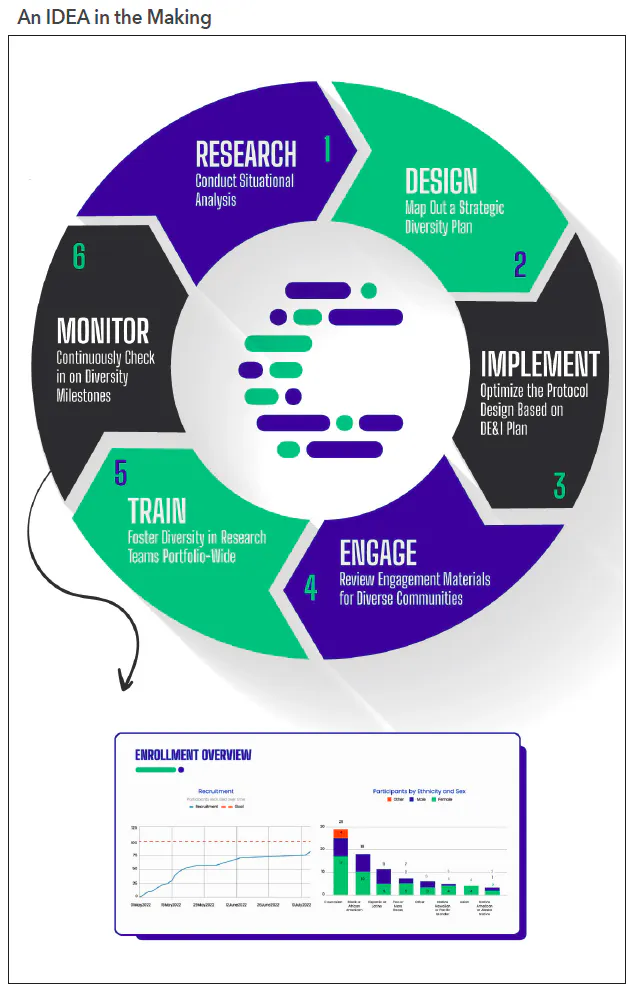
Not sure where to start? The following are six steps that follow Curavit’s inclusivity, diversity, and equity in action (IDEA) toolkit,4 which involves a clinical trial planning and support framework designed to help sponsors navigate the myriad challenges in developing and executing diversity action plans (see Figure 1).
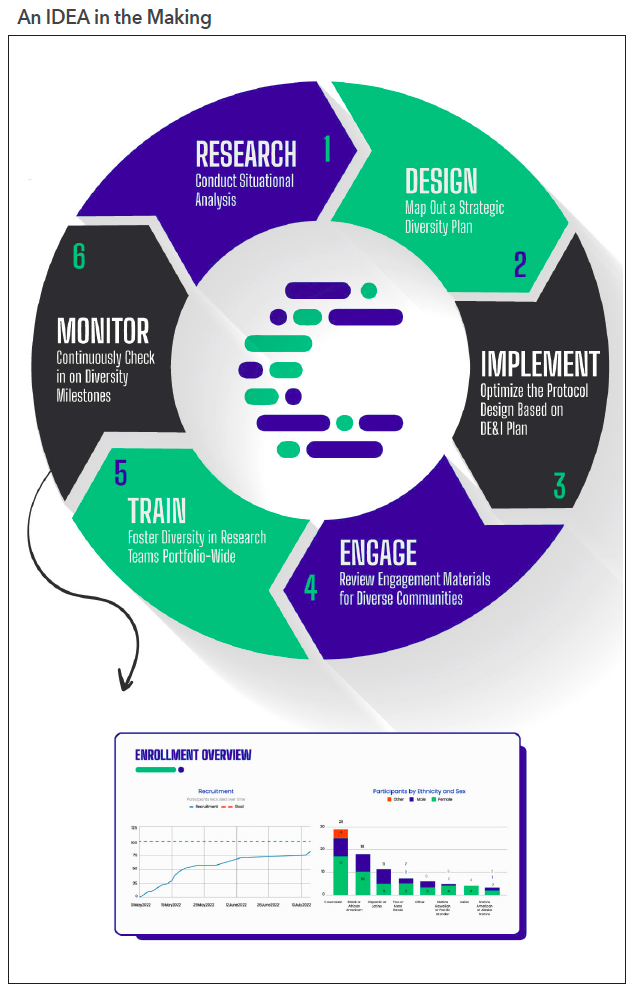
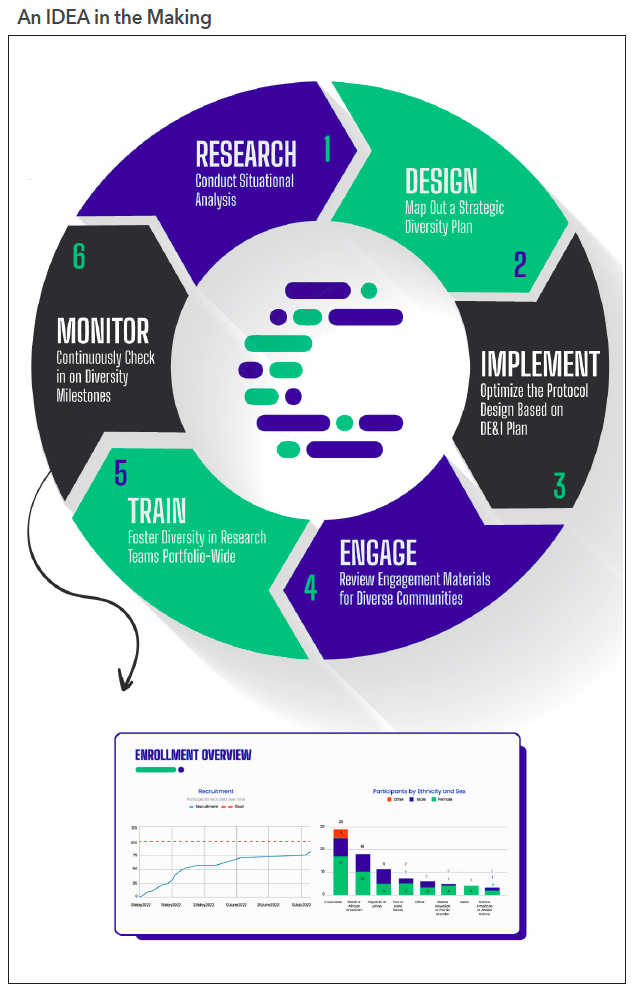
Inclusivity, diversity, and equity in action
1. Research: Conduct situational analysis. Before protocol designing, prepare a summary document consisting of information about the trial’s specific patient-population needs based on therapeutic area, disease risk factors, and potential obstacles to recruitment and retention. Conduct a thorough analysis of the demographics of the target population, looking at the latest census data while overlaying the geographic prevalence of patients with the disease being studied.
Understanding geographic prevalence provides insights on which regions to target for recruitment for investigator sites and patients while also informing any decisions around decentralization. For instance, if the patient population with the highest disease prevalence is clustered in remote areas throughout the southwestern US, an electronic consent tool and telehealth services should be built into the protocol design to reduce potential travel burdens. To understand the prevalence of this disease among different groups, it is useful to review National Institute of Health publications and CDC data.
In most cases, this preliminary research requires a few different data sources overlaid on top of one another to correlate geography with disease prevalence and demographics. Cross-reference multiple data points to ensure the accuracy of that patient population. This will give study staff a composite picture of the situation to better inform the diversity plan.
There is one caveat: because patients are historically underrepresented in research, existing data may not accurately reflect the true prevalence of the disease among minority groups. Until more accurate data is generated, consider over-enrolling across population minority groups.
2. Design: Map out a strategic diversity plan. The key word is “strategic.” The plan should be based on a thorough analysis of the demographics of the target population, including the potential barriers to participation. It must also include realistic recruitment goals, patient engagement, retention strategies, and metrics for measuring success. Seek the assistance of a specialty firm to help create this plan and enroll appropriately representative numbers of participants from underrepresented populations to be submitted with investigational new drug and investigational device applications.
3. Implement: Optimize the protocol design based on the diversity, equity, and inclusion (DE&I) plan. Once the DE&I plan is finalized, optimize the study design, taking into consideration the results of the situational analysis research. This starts with strategic site selection (if using brick-and-mortar sites) in diverse communities or identifying a combination of traditional and alternative or virtual sites that will enable the greatest access to the target patient population.
Also, talk to patient recruitment specialists to uncover innovative and new recruitment methods, technologies, and partners uniquely qualified for reaching the target patient population. If needed, adjust the protocol design to improve patient enrollment and retention across historically underrepresented groups. This may include reconsidering inclusion/exclusion criteria and eliminating any overly strict eligibility criteria.
At this point, too, consider incorporating decentralized methods and technologies into the trial design to improve patient enrollment. DCTs reduce many of the traditional barriers to study participation. For example, a DCT that uses virtual trial sites does not require travel (and the associated expense) for participants, requires less time off work, and offers more flexibility not limited by a medical office’s operating hours.
4. Engage: Review engagement materials for diverse communities. Deciding whether to participate in a clinical trial can, frankly, be a big undertaking. The risks and benefits must be weighed carefully and that is not always easy for those who may not have a high degree of health and science literacy. Having trusted friends, family, and community members to consult about the decision can make all the difference for a hesitant would-be participant. By partnering with local community organizations, leaders, and community medical providers, clinical trial sponsors can spread awareness of their studies through those who are already deeply embedded and trusted in their communities. These community leaders can help build a bridge of familiarity, trust, and understanding between the study team and potential participants.
It’s important to review recruitment content to ensure productive engagement with historically underserved communities. Avoid natural bias by seeking external feedback on the language, design, imagery, and cultural relevance of all patient recruitment marketing materials (i.e., websites, brochures, social media posts). Address any cultural and linguistic barriers to participation by translating study materials into different languages using culturally appropriate visuals and messaging, and providing interpretation services during study visits.
5. Train: Foster diversity in research teams portfolio-wide. Clinical trial sponsors should foster diversity within their research teams. This can include recruiting and promoting researchers from diverse backgrounds, providing training and support for researchers to better understand and work with diverse communities, and ensuring that study teams reflect the diversity of the population being studied. Some studies have even shown lower attrition rates of minority participants when the study teams are from familiar and similar cultural backgrounds.
By promoting DE&I internally, sponsors and sites bring unique perspectives and cultural competencies to trials. For example, a Black female research coordinator noted that a trial participant told her how thankful she was to see someone who looks like her on the study team—“it gives me a sense of community and fosters trust,” she said.
6. Monitor: Continuously check in on diversity milestones. Sponsors should be aware of the changing demographics of the study participant pool throughout the course of the study and be prepared to take steps to address any shifting gaps in representation. Track attrition rates in real time and continuously look to eliminate stress points that might be causing participants to discontinue their involvement with the trial. Technology platforms can provide study teams with near-real-time dashboards to track enrollment progress versus projected milestones to continuously monitor status and optimize as needed.
Managing diversity in clinical research is an ongoing process of fine-tuning, monitoring to see if you are hitting key milestones as outlined in the plan, and refining in a cyclical process for continuous improvement. For example, in a multi-year clinical trial that might involve more than 20,000 participants, staff should be ready to course-correct in expectation of patient attrition, as recent data shows about 18%5 and as high as 38%6 of patients drop out across therapeutic areas and phases. Further analysis5 reveals that sponsors need to identify 10 patients to randomize just one.
Given the variability from trial to trial, there’s no one-size-fits-all approach, technology, or methodology for diversity planning. However, starting with a proven framework can accelerate the planning process, remove uncertainty, and prevent costly missteps when it comes to meeting diversity milestones.
What does the future look like?
With the US government, for the first time in history, codifying into law the responsibility to ensure a representative patient population, it is a landmark moment for the clinical research industry. And while it is a long overdue one, it will take some time to get the new changes right and will require a long-term commitment.
There are no silver bullets nor sustainable quick fixes. Still, the life sciences industry is inching toward a better research environment that looks different than it did a decade ago—and that will benefit all of humanity.
Natalia Husby, Solutions Manager, Curavit Clinical Research