Media Coverage | Contract Pharma
June 13, 2023
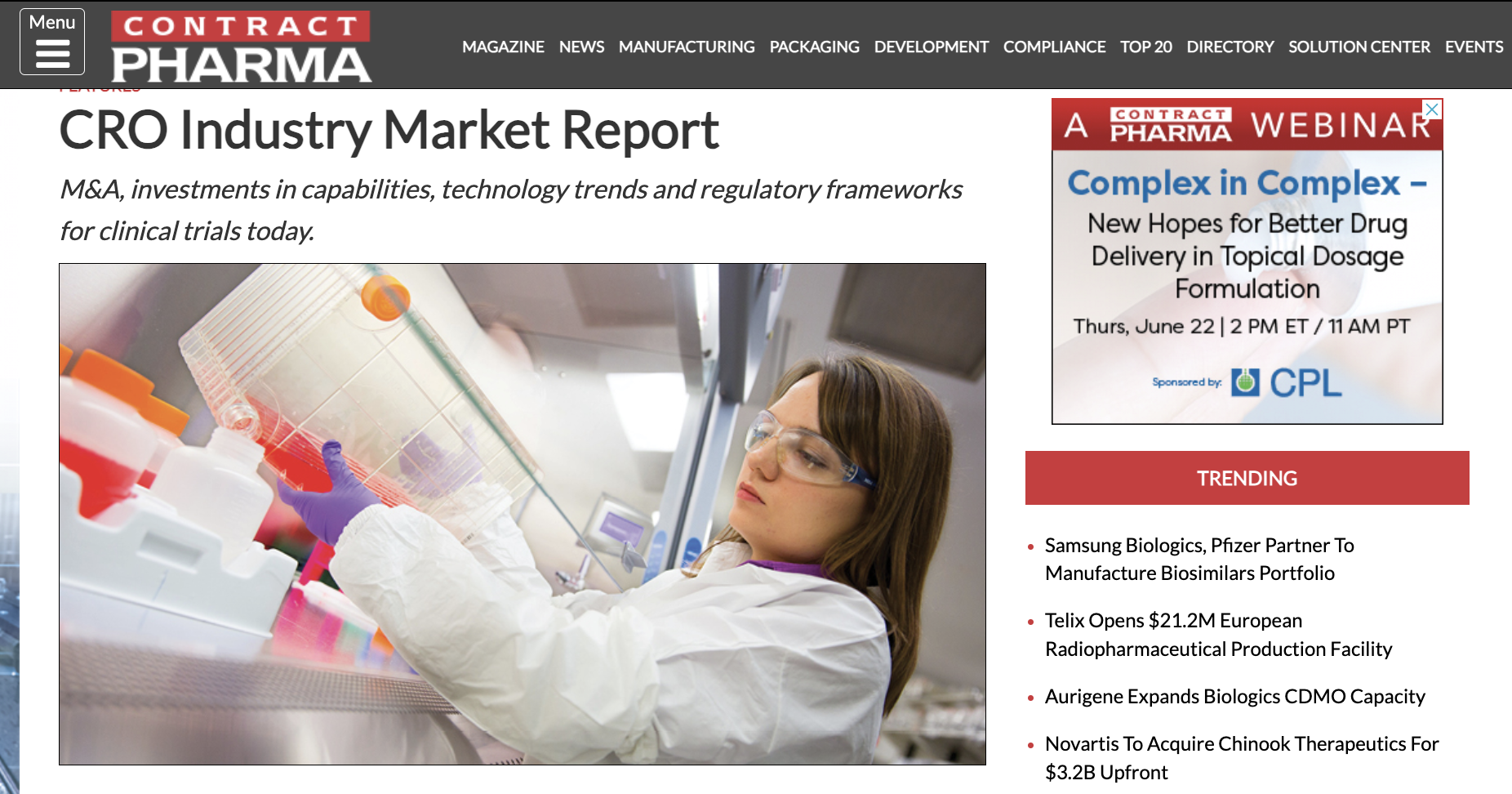
According to Joel Morse, CEO and co-founder of Curavit Clinical Research, several factors influence today’s outsourcing landscape, including available resources, regulatory changes, and advancements in technology.
“With these factors in mind, we are seeing an increased reliance on outsourced expertise in virtual/remote clinical trial design and execution to keep costs down, along with experience in recruiting and enrolling a diverse trial participant population to meet new regulatory requirements and improve patient care for all.”
“It’s a big question and I think you could come at it from many different angles, but if we look at what we see at Emmes as the most promising growth areas then we continue to see good demand for vaccine and infectious diseases, ophthalmology and rare disease—all of which we are well known for,” said Adam Mendizabal, vice president and director of Emmes’ Cell and Gene Therapy Center. “But in the newer areas, then yes, cell and gene therapies continue to be area of considerable growth. What’s most significant is that we now have advanced therapies in development for nearly every single therapeutic area—from oncology through to hematology, ophthalmology, cardiology, along with neurological targets and not to mention a major focus on rare diseases. From a CRO perspective this means we need a wide breadth of clinical indications experience to build the teams for each advanced therapy trial. This is also why you will see CROs building multi-functional Centers of Excellence for cell and gene therapies so that you have these wide skill sets that can be tailored to the unique trial needs.”
Joel Morse
CEO and co-founder of Curavit Clinical Research









