December 14, 2023
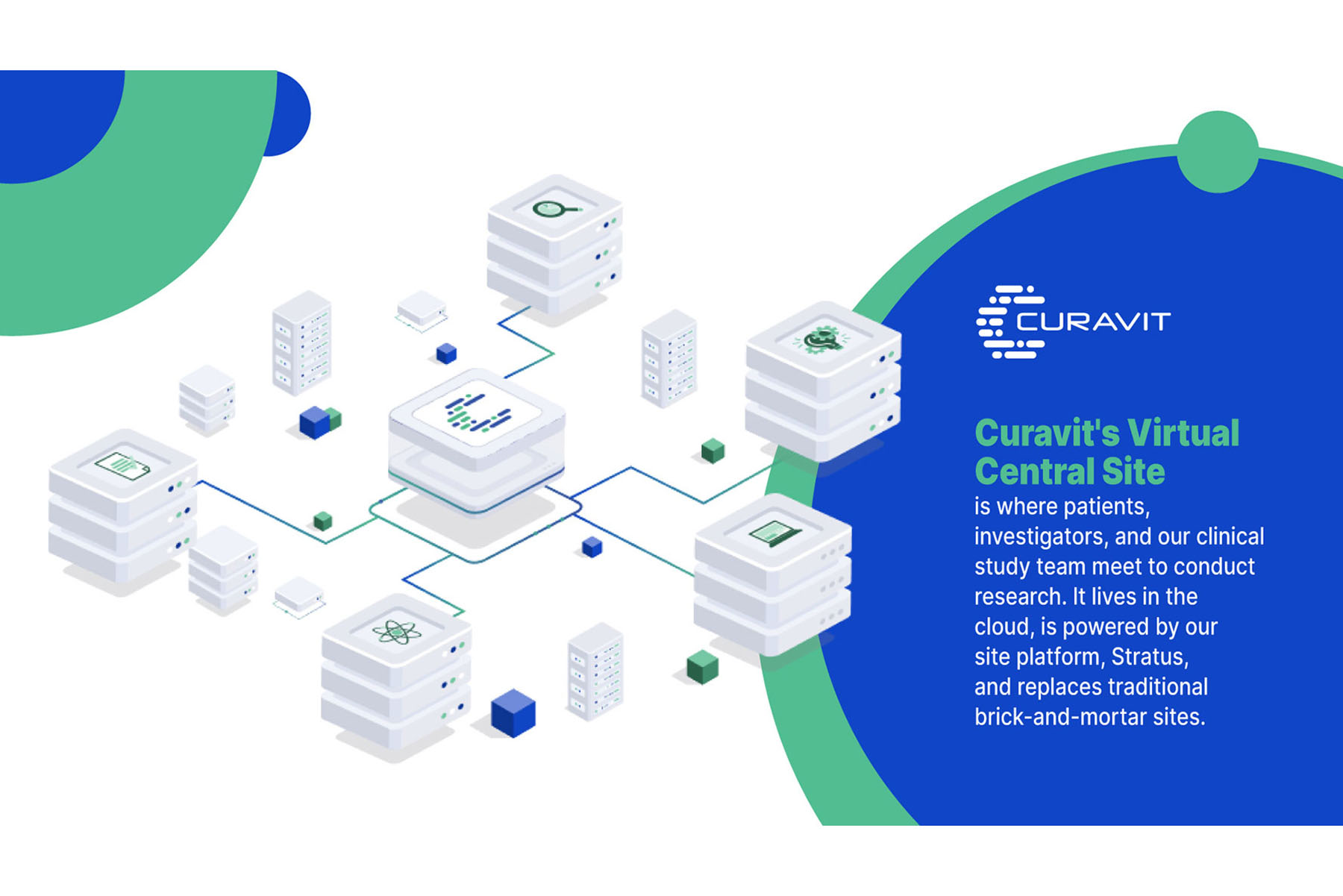
Curavit Clinical Research’s Central Virtual Site
The Advantage of Virtual Clinical Trials
Swing Therapeutics engaged Curavit Clinical Research to act as a virtual site in a pivotal trial of an investigational digital therapeutic (DTx) for fibromyalgia. Curavit’s clinical operations team recruited, screened, consented, and enrolled participants from across the United States in Swing’s PROSPER-FM randomized clinical trial, and subsequently managed the enrolled participants throughout the 12-week study. By leveraging its virtual site, Curavit was able to eliminate physical and geographic barriers to trial participation, scale quickly, and catalyze patient enrollment.
Curavit’s virtual site randomized 10 times more study subjects than traditional sites that started at the same time. More importantly, Curavit’s virtual site achieved the highest level for study subjects’ adherence to protocol.
Swing Therapeutics designed the original protocol for their Prospective Study to Evaluate a Digital Regimen for Fibromyalgia Management (PROSPER-FM) to fully enable enrolled study subjects to participate from home. The “participate from home” protocol design was meant to improve the study participant experience. The protocol was then adjusted to leverage Curavit’s virtual site for cloud-based screening, consent, and enrollment. At the time, Mike Rosenbluth, PhD, Chief Executive Officer at Swing noted: “Curavit’s virtual services model enables us to efficiently recruit participants representative of the entire patient population.”
Curavit’s virtual site supports diverse patient recruitment and removes geographic boundaries to participation without the expense of physical infrastructure. This increases diversity, patient-centricity, and the likelihood that participants will stick with the trial for the duration.